Plagiarism and the role of publishers
04/02/18 18:34
On Friday a colleague made me aware that Giusy and I have been blatantly plagiarized. It's in a book published by Elsevier, called "Nanostructured Polymer Blends" and edited by Sabu Thomas, Robert Shanks and Sarahchandran Chandrasekharakurup, that contains a chapter 9 entitled "Nanostructured Liquid Crystals", supposedly written by Goddeti Siva Mohan Reddy, Jaragula Jayaramudu, Kokkarachedu Varaprasad, Rotimi Sadiku, Shanavas Abdul Jailani and Blessing Atim Aderibigbe. It is interesting that neither I nor any of my colleagues have ever heard of these people, yet they are, according to Elsevier, sufficiently prominent liquid crystal researchers to write a book chapter on liquid crystals! The abstract of that chapter: https://www.sciencedirect.com/science/article/pii/B9781455731596000092
is a word-by-word copy of the abstract of one of Giusy's and my publications (actually, it is my most cited article at present ;-): https://www.sciencedirect.com/science/article/pii/S1567173912001113?via%3Dihub
Our article was published two years earlier in Current Applied Physics, another Elsevier publication.
I first thought that the book was published on some obscure predatory publishing house, but when I saw that it was published by Elsevier I was even more alarmed. Since the University of Luxembourg is subscribing to on-line access to Elsevier e-books, I could download the whole chapter. And of course I then found that the whole chapter is copied. Some simple googling found the following examples.
In section 1, Reddy et al. have the following text on the history of liquid crystals:
"Friedrich Reinitzer, an Austrian botanist, discovered the existence of the liquid crystalline state in 1888 [4]. He noted that cholesteryl benzoate exhibits two melting points: first the crystal melts into a cloudy liquid at 145.5 C and then liquid becomes clear at 178.5 C. On subsequent cooling, he observed similar color effects associated with melt of cholesteryl acetate. First, a pale blue color appeared as the clear liquid turned cloudy and then it turned a bright blue-violet color followed by crystallization [4]. Reinitzer sent the samples of this substance to a German physicist Otto Lehmann who was studying the crystallization properties of various substances [5]. Lehmann observed the sample with a polarizing microscope and noted its similarity to some of the known samples."
Now compare that with the following excerpt from Peter Collings' and Michael Hird's introductory book on liquid crystals, here from a screenshot from a Google Books preview:
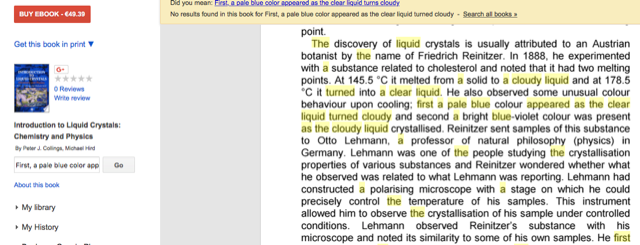
It's not a word-by-word copy, but the similarities are rather striking, are they not? A little bit later, the book chapter writes the following about smectic liquid crystals:
"The lamellar smectic state can be divided into four subgroups by considering the extent of the in-plane positional ordering of the constituent molecules and the tilt orientational ordering of the long axes of the molecules relative to the layer planes. Two groups can be defined where the molecules have their long axes essentially normal to the layers. These two groups are distinguished from each other by the extent of the positional ordering of the constituent molecules. For example, smectic A and hexatic B are smectic liquid crystals in which the molecules have only short-range positional order, whereas crystal B and crystal E are smectic crystal modifications where the molecules have long-range orientational order in three dimensions [17]. Two other classes can be distinguished where the molecules are tilted with respect to the layer planes. In smectic C, smectic I, and smectic F the molecules have short-range orientational ordering, whereas in crystal G, crystal H, crystal J, and crystal K the molecules have long- range three-dimensional ordering [17]. "
It didn't take too much googling to find the source of that either. It's the book "Progress In Liquid Crystal Science And Technology: In Honor of Shunsuke Kobayashi's 80th Birthday", chapter "Defect textures of liquid crystals" by Steven Cowling et al. Here's again the screenshot from the Google Books preview.

Moving to chapter 2 in the Reddy et al. book chapter, where they should be discussing the main topic of the chapter, we find the text: "Recently, their biomedical applications such as in controlled drug delivery, protein binding, phospholipid labeling, and in microbe detection have been demonstrated [34]. Owing to their dynamic nature, photochemically, thermally, or mechanically induced structure changes of liquid crystals can be used for the construction of stimuli-responsive multifunctional materials."
This is taken from Prof. Sandeep Kumar's book "Chemistry of Discotic LIquid Crystals: From Monomers to Polymers":
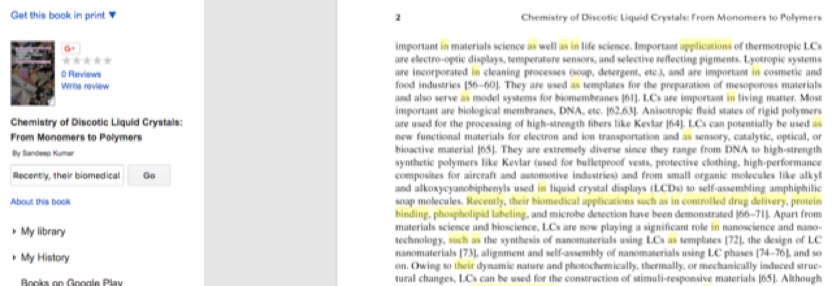
In section 9.2.1, Reddy et al. write:
"Nanoscale particles are generally assumed to be spherical. However, an interesting interplay exists between the surface morphology and the packing arrangement, especially in small nanocrystals. Nanocrystals usually have a polyhedral surface morphology. Nanoparticles also lie in size between that of bulk materials and atoms and provide greater insights into how different properties evolve from atoms to bulk materials and vice versa. The search for the crossover and/or emergence of properties has led to systematic investigation of nanoparticles by both “top- down” and “bottom-up” approaches in nanotechnology. The electronic absorption of nanocrystals of metals is dominated by the surface plasmon resonance (SPR) band, which arises due to the collective coherent excitement of the free electrons within the conduction band. The focus of current research worldwide is to design nanomaterials that are able to self-assemble into functional superstructures in multiple directions. Nanoparticles’ assembly has been induced by several methods, however, using liquid crystals as an organizing medium to induce the self-assembly of nanoparticles is a powerful tool. Since a great variety of mesophase morphologies can be achieved to control the structure in one or more dimensions. The self-organization of nanomaterials in two- and three-dimensional space offered by the LC medium seems to be an ideal vehicle to explore and control the organization of matter on the nanometer to the micrometer scale, which is a key to the further development of nanotechnology. One of the pursued classes of nanomaterials is metal nanoparticles. Gold nanoparticles (GNPs) are likely the best understood (chemical properties and stabilizing techniques) and therefore the most frequently studied nanoparticles. Consequently GNPs have been designed to exhibit liquid-crystalline behavior as well, as they have been dispersed into thermotropic and lyotropic liquid-crystalline phases, to modify the properties of the hosts [39]."
Please compare this with the following excerpt from the tutorial review "Liquid-crystal nanoscience: an emerging avenue of soft self-assembly", published in Chem. Soc. Rev. by Bosoyi and Kumar:
"Nanoscale particles are generally assumed to be spherical. However, an interesting interplay exists between the surface morphology and the packing arrangement, especially in small nanocrystals. Nanocrystals usually have a polyhedral surface morphology. Nanoparticles also lie in size between that of bulk materials and atoms and provide greater insights into how different properties evolve from atoms to bulk materials and vice versa. The search for the cross-over and/or emergence of properties have led to systematic investigation of nanoparticles by both ‘top-down’ and ‘bottom-up’ approaches in nanotechnology. The electronic absorption of nanocrystals of metals is dominated by the surface plasmon resonance (SPR) band which arises due to the collective coherent excitement of the free electrons within the conduction band. The focus of current research worldwide is to design nanomaterials that are able to self-assemble into functional superstructures in multiple directions. Nano- particles assembly has been induced by several methods; however, using liquid crystals as an organizing medium to induce the self-assembly of nanoparticles is a powerful tool. Since a great variety of mesophase morphologies can be achieved to control the structure in one or more dimensions. The self-organization of nanomaterials in two- and three- dimensional space offered by the LC medium seems to be an ideal vehicle to explore and control the organization of matter on the nanometre to the micrometre scale which is a key to the further development of nanotechnology. One of the pursued classes of nanomaterials is metal nanoparticles. Gold nano- particles (GNPs) are likely the best understood (chemical properties and stabilizing techniques) and therefore the most frequently studied nanoparticles. Consequently GNPs have been designed to exhibit liquid-crystalline behavior as well, as they have been dispersed into thermotropic and lyotropic liquid-crystalline phases, to modify the properties of the hosts."
The whole chapter is an act of blatant plagiarism from beginning to end. On Friday I wrote to Elsevier and asked them to retract the chapter and take action against the authors, so far only with some computer-generated auto reply. I certainly hope that they will react quickly, because this is really serious. There needs to be criminal charges taken for cases like this, because otherwise this will just get worse. I know, of course, that there are thousands of similar examples.
What shocks me the most is that Elsevier is publishing this book. Apparently they do zero plagiarism check on their own publications!!! And since in this case the plagiarizing book chapter has an abstract that is taken from an article published by the same publisher as the book, we are in the interesting copyright law situation that Elsevier has the copyright to both the original and the copy! Who should be suing who here?
It'll be interesting to see how Elsevier acts and responds. I'll update this page when there are news.
is a word-by-word copy of the abstract of one of Giusy's and my publications (actually, it is my most cited article at present ;-): https://www.sciencedirect.com/science/article/pii/S1567173912001113?via%3Dihub
Our article was published two years earlier in Current Applied Physics, another Elsevier publication.
I first thought that the book was published on some obscure predatory publishing house, but when I saw that it was published by Elsevier I was even more alarmed. Since the University of Luxembourg is subscribing to on-line access to Elsevier e-books, I could download the whole chapter. And of course I then found that the whole chapter is copied. Some simple googling found the following examples.
In section 1, Reddy et al. have the following text on the history of liquid crystals:
"Friedrich Reinitzer, an Austrian botanist, discovered the existence of the liquid crystalline state in 1888 [4]. He noted that cholesteryl benzoate exhibits two melting points: first the crystal melts into a cloudy liquid at 145.5 C and then liquid becomes clear at 178.5 C. On subsequent cooling, he observed similar color effects associated with melt of cholesteryl acetate. First, a pale blue color appeared as the clear liquid turned cloudy and then it turned a bright blue-violet color followed by crystallization [4]. Reinitzer sent the samples of this substance to a German physicist Otto Lehmann who was studying the crystallization properties of various substances [5]. Lehmann observed the sample with a polarizing microscope and noted its similarity to some of the known samples."
Now compare that with the following excerpt from Peter Collings' and Michael Hird's introductory book on liquid crystals, here from a screenshot from a Google Books preview:

It's not a word-by-word copy, but the similarities are rather striking, are they not? A little bit later, the book chapter writes the following about smectic liquid crystals:
"The lamellar smectic state can be divided into four subgroups by considering the extent of the in-plane positional ordering of the constituent molecules and the tilt orientational ordering of the long axes of the molecules relative to the layer planes. Two groups can be defined where the molecules have their long axes essentially normal to the layers. These two groups are distinguished from each other by the extent of the positional ordering of the constituent molecules. For example, smectic A and hexatic B are smectic liquid crystals in which the molecules have only short-range positional order, whereas crystal B and crystal E are smectic crystal modifications where the molecules have long-range orientational order in three dimensions [17]. Two other classes can be distinguished where the molecules are tilted with respect to the layer planes. In smectic C, smectic I, and smectic F the molecules have short-range orientational ordering, whereas in crystal G, crystal H, crystal J, and crystal K the molecules have long- range three-dimensional ordering [17]. "
It didn't take too much googling to find the source of that either. It's the book "Progress In Liquid Crystal Science And Technology: In Honor of Shunsuke Kobayashi's 80th Birthday", chapter "Defect textures of liquid crystals" by Steven Cowling et al. Here's again the screenshot from the Google Books preview.

Moving to chapter 2 in the Reddy et al. book chapter, where they should be discussing the main topic of the chapter, we find the text: "Recently, their biomedical applications such as in controlled drug delivery, protein binding, phospholipid labeling, and in microbe detection have been demonstrated [34]. Owing to their dynamic nature, photochemically, thermally, or mechanically induced structure changes of liquid crystals can be used for the construction of stimuli-responsive multifunctional materials."
This is taken from Prof. Sandeep Kumar's book "Chemistry of Discotic LIquid Crystals: From Monomers to Polymers":

In section 9.2.1, Reddy et al. write:
"Nanoscale particles are generally assumed to be spherical. However, an interesting interplay exists between the surface morphology and the packing arrangement, especially in small nanocrystals. Nanocrystals usually have a polyhedral surface morphology. Nanoparticles also lie in size between that of bulk materials and atoms and provide greater insights into how different properties evolve from atoms to bulk materials and vice versa. The search for the crossover and/or emergence of properties has led to systematic investigation of nanoparticles by both “top- down” and “bottom-up” approaches in nanotechnology. The electronic absorption of nanocrystals of metals is dominated by the surface plasmon resonance (SPR) band, which arises due to the collective coherent excitement of the free electrons within the conduction band. The focus of current research worldwide is to design nanomaterials that are able to self-assemble into functional superstructures in multiple directions. Nanoparticles’ assembly has been induced by several methods, however, using liquid crystals as an organizing medium to induce the self-assembly of nanoparticles is a powerful tool. Since a great variety of mesophase morphologies can be achieved to control the structure in one or more dimensions. The self-organization of nanomaterials in two- and three-dimensional space offered by the LC medium seems to be an ideal vehicle to explore and control the organization of matter on the nanometer to the micrometer scale, which is a key to the further development of nanotechnology. One of the pursued classes of nanomaterials is metal nanoparticles. Gold nanoparticles (GNPs) are likely the best understood (chemical properties and stabilizing techniques) and therefore the most frequently studied nanoparticles. Consequently GNPs have been designed to exhibit liquid-crystalline behavior as well, as they have been dispersed into thermotropic and lyotropic liquid-crystalline phases, to modify the properties of the hosts [39]."
Please compare this with the following excerpt from the tutorial review "Liquid-crystal nanoscience: an emerging avenue of soft self-assembly", published in Chem. Soc. Rev. by Bosoyi and Kumar:
"Nanoscale particles are generally assumed to be spherical. However, an interesting interplay exists between the surface morphology and the packing arrangement, especially in small nanocrystals. Nanocrystals usually have a polyhedral surface morphology. Nanoparticles also lie in size between that of bulk materials and atoms and provide greater insights into how different properties evolve from atoms to bulk materials and vice versa. The search for the cross-over and/or emergence of properties have led to systematic investigation of nanoparticles by both ‘top-down’ and ‘bottom-up’ approaches in nanotechnology. The electronic absorption of nanocrystals of metals is dominated by the surface plasmon resonance (SPR) band which arises due to the collective coherent excitement of the free electrons within the conduction band. The focus of current research worldwide is to design nanomaterials that are able to self-assemble into functional superstructures in multiple directions. Nano- particles assembly has been induced by several methods; however, using liquid crystals as an organizing medium to induce the self-assembly of nanoparticles is a powerful tool. Since a great variety of mesophase morphologies can be achieved to control the structure in one or more dimensions. The self-organization of nanomaterials in two- and three- dimensional space offered by the LC medium seems to be an ideal vehicle to explore and control the organization of matter on the nanometre to the micrometre scale which is a key to the further development of nanotechnology. One of the pursued classes of nanomaterials is metal nanoparticles. Gold nano- particles (GNPs) are likely the best understood (chemical properties and stabilizing techniques) and therefore the most frequently studied nanoparticles. Consequently GNPs have been designed to exhibit liquid-crystalline behavior as well, as they have been dispersed into thermotropic and lyotropic liquid-crystalline phases, to modify the properties of the hosts."
The whole chapter is an act of blatant plagiarism from beginning to end. On Friday I wrote to Elsevier and asked them to retract the chapter and take action against the authors, so far only with some computer-generated auto reply. I certainly hope that they will react quickly, because this is really serious. There needs to be criminal charges taken for cases like this, because otherwise this will just get worse. I know, of course, that there are thousands of similar examples.
What shocks me the most is that Elsevier is publishing this book. Apparently they do zero plagiarism check on their own publications!!! And since in this case the plagiarizing book chapter has an abstract that is taken from an article published by the same publisher as the book, we are in the interesting copyright law situation that Elsevier has the copyright to both the original and the copy! Who should be suing who here?
It'll be interesting to see how Elsevier acts and responds. I'll update this page when there are news.
blog comments powered by Disqus